 Review Article
Review Article
Theoretical Investigation on [2 + 2]/ [3 + 2] Cycloaddition from Biphosphole and Nitrone Yielding Phospholene Fused β‑Phosphinolactam or Isoxazolidine
Nan Lu* and Chengxia Miao
College of Chemistry and Material Science, Shandong Agricultural University, PR China
Lu, College of Chemistry and Material Science, Shandong Agricultural University, Taian 271018, PR China
Received Date:October 26, 2024; Published Date:November 08, 2024
Abstract
Our DFT calculations provide the first theoretical investigation on [2 + 2] and [3 + 2] cycloaddition from biphosphole and nitrone. The dissociation of biphosphole generates 1-phosphafulvene. The redox be-tween 1-phosphafulvene and nitrone provides 1-phosphafulvene oxide and imine. Via oxidation, the reactiv-ity is boosted facilitating nucleophilic addition. The [2 + 2] cycloaddition produces phospholene fused β-phosphinolactam as major product with quaternary ring. A competitive path exists owing to antiaromaticity of phosphole oxide. The negative ion shifting to exocyclic carbon prompts intramolecular nucleophilic addition of carbanion to iminium giving another phosphole oxide with five-membered ring. With a second nitrone, [3 + 2] cycloaddition yields phospholene fused isoxazolidine as by-product with two five-membered rings. The positive solvation effect is suggested by decreased absolute and activation energies in solution compared with in gas. These results are supported by Multiwfn analysis on FMO composition of specific TSs, and MBO value of vital bonding, breaking.
Abbreviations: [2 + 2] cycloaddition; 1-phosphafulvene; biphosphole; nitrone; β-phosphinolactam
Introduction
As privileged precursor of various novel P-heterocycles, 2H-phosphole presents versatile cyclopentadi-ene-like reactivity owing to the structural components of highly reactive C=P bond [1]. Based on this chemistry, 2H-phosphole usually acts as 2π and 4π component with electron-deficient 1,3-dipole (nitrile oxide), dienes and in reaction with dienophiles. For instance, Mathey discovered transient 2H-phospholes as powerful syn-thetic intermediates in organophosphorus chemistry [2]. Wonneberger realized access to 1-phospha-2-azanorbornenes by phosphaaza- Diels−Alder reaction [3]. Zhang researched the synthesis and functionalization of 6-methylene-1-phosphanorbornenes in hetero-Diels−Alder reaction of 2H-phospholes with allenes [4]. On the contrary of stable double bond in ketone, imine and alkene, the introduction of heav-ier elements cause extreme unstable just as Weetman reported main group multiple bonds for bond activation and catalysis [5]. In this field, many processes have been achieved in synthesis of a series of P-stereogenic ligands [6-10]. Compared with 2H-phosphole, 1-Phosphafulvene has exocyclic C=C double bond displaying more cycload-dition chemistry as one versatile phospha-analogue of pentafulvene. Hu obtained one-step synthesis of che-lating, alpha-C2-bridged biphospholes in reaction of phospholes with aldimines [11]. Then this group proved that 1-phosphafulvene can participate in reversible [6 + 6] dimerization as 2π, 4π and 6π components to give [6 + 4] cycloadduct [12]. In [2 + 4] cycloaddition reaction, 1-phosphafulvene acts as electronpoor partner toward conjugated dienes for synthesis of polycyclic phosphacycles [13]. Recently, Liu group developed 1,1-addition of biphospholes with alkynes, FeCl2 catalyzed three-component reaction of phospholes, pyrroli-dine, ketones [14,15] and explored nonbenzenoid aromaticity of 1-phosphafulvenes in synthesis of phos-phacymantrenes [16]. On the other hand, β-Lactams are clinically vital antimicrobials in human and veterinary medicine. However, only limited attention paid on their phosphorus analogues such as Afarinkia’s 1,2-azaphosphetidines, a broad-spectrum inhibitor of metallo-β-lactamases prepared by Yang as carbapenem transition state analog, 1,2-azaphosphetidine 2-oxides/sulfides of Xu and β-phosphinolactams from phos-phenes/imines discovered by Fu [17-20].
In recent years, [3 + 2] cycloaddition gained attention including metal-free diastereo- and enantioselective dearomative formal version of 2-nitrobenzofurans and isocyanoacetate esters of Laviós [21]. He also found diastereoselective synthesis of dihydrobenzofuran-fused spiroindolizidines via double- Dearomative mode [22]. Aspired by this, Tian group prepared phospholene fused isoxazolidine through [3 + 2] cycloaddition of phosphole sulfides and nitrones [23]. A breakthrough was reaction of α-C2–bridged biphosphole as precur-sor of 1-phosphafulvene toward nitrone [24]. Although a range of phospholene fused β-phosphinolactams were yielded, many problems still puzzled and there was no report about detailed mechanistic study explaining the slight antiaromaticity of phosphole oxide suggested previously [25]. How the reactivity of 1-phosphafulvene is boosted via oxidation? Since 1-phosphafulvene oxide is unstable, how to verify the nu-cleophilic addition of imine to it? Given antiaromaticity disfavoring negative ion migration, what’s the me-chanic discrepancy between [2 + 2] and [3 + 2] cycloaddition affording phospholene fused β-phosphinolactam and isoxazolidine respectively? To solve these questions in experiment, an in-depth theoretical study was necessary also focusing on the aromaticantiaromatic switch in P-heteroles.
Computational details
Optimized structures were obtained at M06-2X/6-31G(d) level of theory with GAUSSIAN09 [26]. In tests of popular DFT methods [27], M06-2X functional attained smaller standard deviation of difference between calculated value and experimental value in geometries than B3LYP including Becke’s three-parameter hybrid functional combined with Lee−Yang−Parr correction for correlation [28,29]. The best compromise between accuracy and time consumption was provided with 6-31G(d) basis set on energy calculations. Also, M06-2X functional was found to give relatively accurate results for catalysed enantioselective (4 + 3), concerted [4 + 2], stepwise (2 + 2) cycloaddition and catalysed Diels−Alder reactions [30,31]. Together with the best performance on noncovalent interaction, M06-2X functional is believed to be suitable for this system [32-34]. The nature of each structure was verified by performing harmonic vibrational frequency calculations. Intrinsic reaction coordinate (IRC) calculations were examined to confirm the right connections among key transition-states and corresponding reactants and products. Harmonic frequency calculations were carried out at the M06-2X/6-31G(d) level to gain zero-point vibrational energy (ZPVE) and thermodynamic corrections at 433 K and 1 atm for each structure in toluene.
The solvation-corrected free energies were obtained at the M06-2X/6-311++G(d,p) level by using integral equation formalism polarizable continuum model (IEFPCM) in Truhlar’s “density” solvation model [35-39] on the M06-2X/6-31G(d)-optimized geometries. As an efficient method obtaining bond and lone pair of a molecule from modern ab initio wave functions, NBO procedure was performed with Natural bond orbital (NBO3.1) to characterize electronic properties and bonding orbital interactions [40-42]. The wave function analysis was provided using Multiwfn_3.7_dev package [43] including research on frontier molecular orbital (FMO) and Mayer bond order (MBO).
Results and Discussion
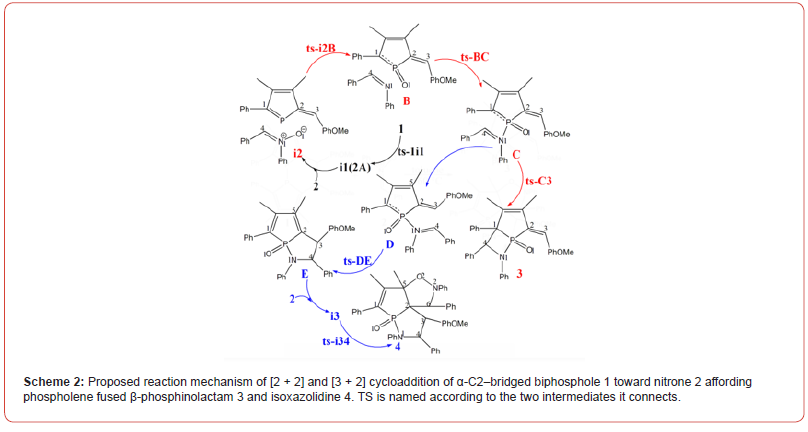
The mechanism was explored for [2 + 2] and [3 + 2] cycloaddition of α-C2–bridged biphosphole 1 toward nitrone 2 affording phospholene fused β-phosphinolactam 3 and isoxazolidine 4 (Scheme 1). Illustrated by black arrow of Scheme 2, the dissociation of biphosphole 1 generates two molecules of 1-phosphafulvene A at first. Then the redox reaction between A and nitrone 2 provides 1-phosphafulvene oxide B and imine (red arrow). The reactivity of A is boosted via oxidation toward imine facilitating the next nucleophilic addition of imine to B affording intermediate C. A formal [2 + 2] cycloaddition of C produces phospholene fused β-phosphinolactam 3 as major product. Another competitive path also exists owing to slight antiaromaticity of phosphole oxide (blue arrow). The negative ion of C shifts to exocyclic carbon atom resulting in intermediate D, from which the intramolecular nucleophilic addition of carbanion to iminium ion gives another phosphole oxide E. The subsequent [3 + 2] cycloaddition between E and a second molecule of nitrone 2 leads to phospholene fused isoxazolidine 4 as by-product. Induced by antiaromaticity, the instability of phosphole oxide disfavors negative ion migration from C to D bringing about low yield of 4. The schematic structures of optimized TSs in Scheme 2 were listed by Figure 1. The activation energy was shown in Table 1 for all steps. Supplementary Table S1, Table S2 provided the relative energies of all stationary points. According to experiment, the Gibbs free energies in toluene solution phase are discussed here.
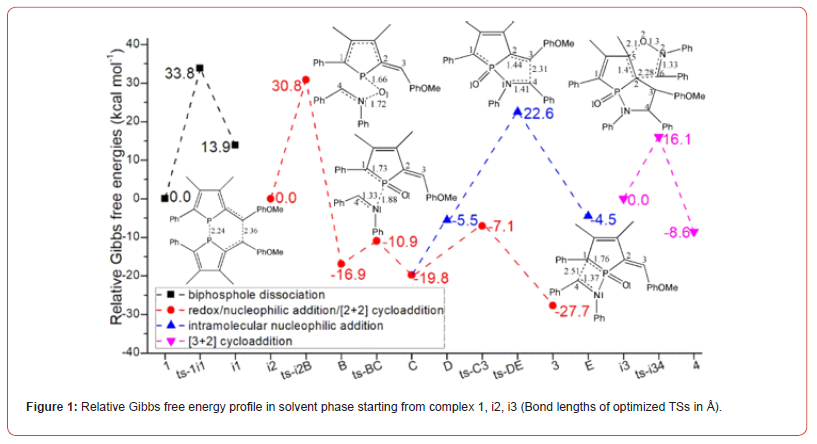
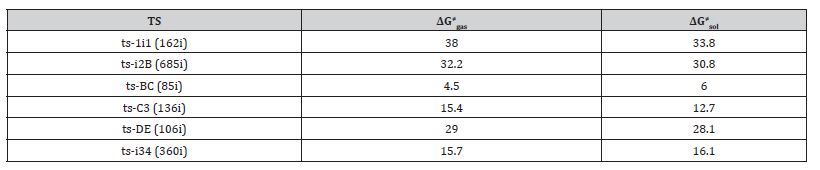
Table 1:The activation energy (in kcal mol−1) of all reactions in gas and solvent.
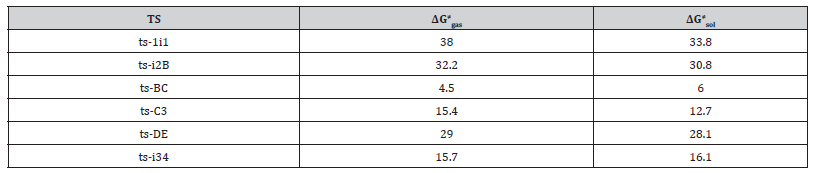
Table S1:Calculated relative energies (all in kcal mol-1, relative to isolated species) for the ZPE-corrected Gibbs free energies (ΔGgas), Gibbs free energies for all species in solution phase (ΔGsol) at 433 K by M06-2X/6-311++G(d,p)//M06-2X/6-31G(d) method and difference between absolute energy.
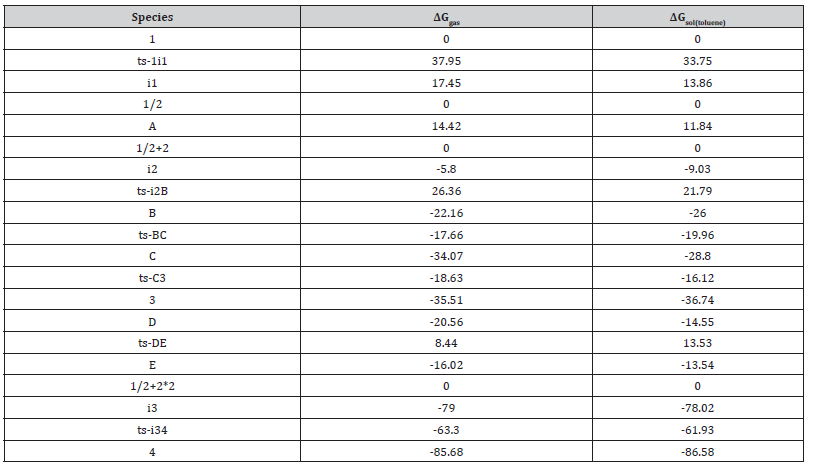
Table S2:The activation energy (local barrier) (in kcal mol−1) of all reactions in the gas, solution phase calculated with M06-2X/6-311++G(d,p)//M06-2X/6-31G(d) method.
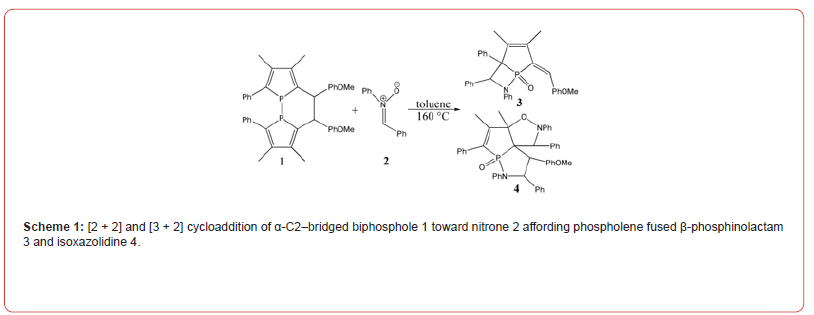
Biphosphole dissociation/redox/nucleophilic addition/ [2 + 2] cycloaddition
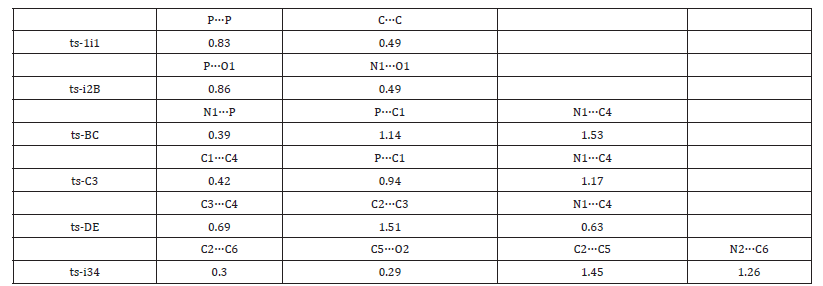
As convenient precursor, the α-C2-bridged biphosphole 1 undergoes dissociation via ts-1i1 in step 1 with the activation energy of 33.8 kcal mol−1 endothermic by 13.9 kcal mol−1 producing reactive complex i1 binding two molecules of 1-phosphafulvene A (black dash line of Figure 1). The transition vector includes simultaneous cleavage of P-P and C-C single bond (2.24, 2.36 Å). The typical structure A is also reactive from its relative energy (11.8 kcal mol−1) compared with the half value of 1, which is ready for the process afterwards.
Then the participation of nitrone 2 with A forms i2 taken as new starting point of next three steps (red dash line of Figure 1). The redox reaction proceeds via ts-i2B in step 2 with activation energy of 30.8 kcal mol−1 exothermic by -16.9 kcal mol−1 generating stable 1-phosphafulvene oxide B and imine. The transition vector suggests breaking down of N1-O1 ionic bond and concerted bonding of P-O1 (1.72, 1.66 Å) (Figure S1a), which is strengthened to P=O1 double bond together with newly formed N1=C4 imine bond in resultant B.
Through oxidation of P, the reactivity of A is further boosted toward imine N1. Thus, the following nucleophilic addition occurs via ts-BC in step 3 with low activation energy of 6.0 kcal mol−1 affording intermediate C exothermic by -19.8 kcal mol−1. The transition vector is complicated containing a series of atomic motion. On one hand, the attack from N1 to P is noticable. On the other, the P-C1 and N1=C4 are slightly weakened (1.88, 1.73, 1.33 Å). As an explanation for experiment, the step from B to C is verified not only readily accessible from kinetics but favorable in thermodynamics [24].
Subsequently, a formal [2 + 2] cycloaddition of C takes place via ts-C3 in step 4 with activation energy of 12.7 kcal mo−1 exothermic by -27.7 kcal mol−1 delivering phospholene fused β-phosphinolactam 3 as major product. The transition vector corresponds to the approaching of carbanion C1 to C4 and continuous stretching of P-C1 and N1-C4 to single bond (2.51, 1.76, 1.37 Å) (Figure S1b). Once typical C1-C4 single bond is formed, the closure of stable quaternary ring is realized involving sp3 hybrid C1 and C4 as well as disappeared positive charge on imine N1. Comparatively, the redox of 1-phosphafulvene in step 2 forming its oxide and imine is determined to be rate-limiting for [2 + 2] cycloaddition.
Intramolecular nucleophilic addition/ [3 + 2] cycloaddition
Owing to the slight antiaromaticity of phosphole oxide, another competitive path is also possible (blue dash line of Figure 1). The negative charge on C1 of C shifts to exocyclic carbon C3 resulting in intermediate D. Hence the intramolecular nucleophilic addition of carbanion C3 to C4 happens via ts-DE in step 5 with activation energy of 28.1 kcal mol−1 exothermic by -4.5 kcal mol−1 giving another phosphole oxide E. The transition vector reveals detailed atomic motion about bonding from C3 to C4 and stretching of C2-C3, N1-C4 to single bond (2.31, 1.44, 1.41 Å) (Figure S1c). The positive charge on iminium ion is also dissolved in new five-membered ring of E. Although the relative energy of D is increased by 14.3 kcal mol−1 from that of C, this path can’t be excluded considering the barrier completely capable to overcome under high temperature in experiment. The uphill energy from C to D agrees well with the case of disfavoring negative ion migration from C to D induced by antiaromaticity and instability of phosphole oxide [24].
Finally, with a second molecule of nitrone 2 and E, the intermediate i3 is located as starting point of last step 6 (magenta dash line of Figure 1). Via ts-i34, [3 + 2] cycloaddition proceeds with mediate activation energy of 16.1 kcal mol−1 exothermic by -8.6 kcal mol−1 yielding another five-membered heterocycle of phospholene fused isoxazolidine 4 as by-product. This process is illustrated according to the transition vector composed of stretching of C2-C5 from double bond to single, linkage of C2-C6 and C5-O2 in concerted mode (1.4, 2.28, 2.10 Å) (Figure S1d). There are also cooperated elongation of N2-O2 and N2-C6. The relative energy of 4 higher by 19.1 kcal mol−1 than that of 3 is in accordance with fact of lower yield for compound 4 than 3 in experiment [24].
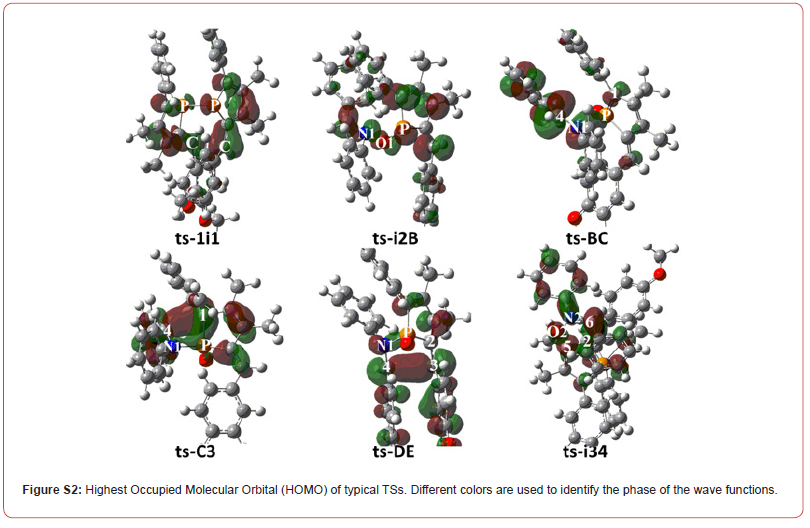
To highlight the idea of feasibility for changes in electron density and not molecular orbital interactions are responsible of the reactivity of organic molecules, quantum chemical tool Multiwfn was applied to analyze of electron density such as MBO results of bonding atoms and contribution of atomic orbital to HOMO of typical TSs (Table S3, Figure S2). These results all confirm the above analysis.
Table S3:Mayer bond order (MBO) of typical TSs.
Conclusions
Our DFT calculations provide the first theoretical investigation on [2 + 2] and [3 + 2] cycloaddition from α-C2-bridged biphosphole and nitrone. As convenient precursor, the dissociation of biphosphole generates two 1-phosphafulvene A. The redox between A and nitrone provides 1-phosphafulvene oxide B and imine. Via oxidation of P, the reactivity of A is boosted toward imine N. This facilitates next nucleophilic addition of imine to B affording C, from which [2 + 2] cycloaddition produces phospholene fused β-phosphinolactam as major product with quaternary ring. The redox of A is rate-limiting for [2 + 2] cycloaddition. A competitive path exists owing to antiaromaticity of phosphole oxide. The negative ion of C shifts to exocyclic carbon resulting in D. The intramolecular nucleophilic addition of carbanion to iminium gives another phosphole oxide E with five-membered ring. The [3 + 2] cycloaddition between E and a second nitrone leads to phospholene fused isoxazolidine as by-product with two five-membered rings. The lower yield of by-product is induced by antiaromaticity of phosphole oxide disfavoring negative ion migration. The positive solvation effect is suggested by decreased absolute and activation energies in toluene solution compared with in gas. These results are supported by Multiwfn analysis on FMO composition of specific TSs, and MBO value of vital bonding, breaking.
Electronic Supplementary Material
Supplementary data available: [Computation information and cartesian coordinates of stationary points; Cal-culated relative energies for the ZPE-corrected Gibbs free energies (ΔGgas), and Gibbs free energies (ΔGsol) for all species in solution phase at 433 K.]
Author Contributions
Conceptualization, Nan Lu; Methodology, Nan Lu; Software, Nan Lu; Validation, Nan Lu; Formal Analysis, Nan Lu; Investigation, Nan Lu; Resources, Nan Lu; Data Curation, Nan Lu; Writing- Original Draft Preparation, Nan Lu; Writing-Review & Editing, Nan Lu; Visualization, Nan Lu; Supervision, Chengxia Miao; Project Administration, Chengxia Miao; Funding Acquisition, Chengxia Miao. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (21972079) and Key La-boratory of Agricultural Film Application of Ministry of Agriculture and Rural Affairs, P.R. China.
Conflict of Interest
The authors declare no conflict of interest.
References
- Mathey F (1992) Expanding the analogy between phosphorus-carbon and carbon-carbon double bonds. Acc Chem Res 25: 90-96.
- Mathey F (2004) Transient 2H-Phospholes as Powerful Synthetic Intermediates in Organophosphorus Chem-istry. Acc Chem Res 37: 954-960.
- Wonneberger P, König N, Kraft FB, Sárosi MB, Hey Hawkins E (2019) Access to 1-Phospha-2-azanorbornenes by Phospha-aza-Diels-Alder Reactions. Angew Chem Int. Ed 58: 3208-3211.
- Zhang K, Zhang Q, Wei D, Tian R, Duan Z (2021) Hetero-Diels-Alder reactions of 2H-phospholes with al-lenes: synthesis and functionalization of 6-methylene-1-phosphanorbornenes. Org Chem Front 8: 3740-3745.
- Weetman C (2021) Main Group Multiple Bonds for Bond Activations and Catalysis. Chem Eur J 27: 1941-1954.
- Möller T, Sárosi MB, Hey Hawkins E (2012) Asymmetric Phospha-Diels-Alder Reaction: A Stereoselective Approach towards P-Chiral Phosphanes through Diastereotopic Face Differentiation. Chem Eur J 18: 16604-16607.
- Möller T, Wonneberger P, Sárosi MB Coburger P, Hey Hawkins E (2016) P-chiral 1-phosphanorbornenes: from asymmetric phospha-Diels-Alder reactions towards ligand design and functionalization. Dalton Trans 45: 1904-1917.
- Gan Z, Zhi M, Han R, Li EQ, Duan Z, Mathey F (2019) P-Stereogenic Phosphines Directed Cop-per(I)-Catalyzed Enantioselective 1,3-Dipolar Cycloadditions. Org Lett 21: 2782-2785.
- Wang Y, Li EQ, Duan Z (2022) Ligand-dependent, palladium-catalyzed stereodivergent synthesis of chiral tetrahy-droquinolines. Chem Sci 13: 8131-8136.
- Meng Q, Meng Y, Liu Q, Yu B, Li ZJm et al. (2024) Enantioselective Synthesis of Oxazocines via MQ-Phos Enabled Palladium-Catalyzed Asymmetric Formal [4 + 4]-Cycloadditions. Adv Sci 11: 2402170.
- Hu Z, Li Z, Zhao K, Tian R, Duan Z, Mathey F (2015) Reaction of Phospholes with Aldimines: A One-Step Synthesis of Chelating, Alpha-C 2 -Bridged Biphospholes. Org Lett 17: 3518-3520.
- Hu Z, Tian R, Zhao K, Liu Y, Duan Z, Mathey F (2017) Generation and Trapping of a 1-Phosphafulvene: An Illustration of the P = C/C = C Analogy. Org Lett 19: 5004-5006.
- Shen N, Liu Y, Tian R, Duan Z, Mathey F (2019) Synthesis of Polycyclic Phosphacycles via 1-Phosphafulvene. Chin J Org Chem 39: 2277-2286.
- Liu Y, Zhang K, Tian R, Duan Z, Mathey F (2020) 1,1-Addition of α-C 2 -Bridged Biphospholes with Al-kynes. Org Lett 22: 6972-6976.
- Liu Y, Fan X, Tian R, Duan Z (2021) FeCl 2 Catalyzed Three-Component Reactions of Phospholes, Pyrroli-dine, and Ketones (Aldehydes): Chemoselective Synthesis of 1-Phosphafulvenes. Org Lett 23: 2943-2947.
- Liu Y, Tian R, Duan Z, Mathey F (2021) Nonbenzenoid aromaticity of 1-phosphafulvenes: synthesis of phosphacymantrenes. Dalton Trans 50: 476-479.
- Afarinkia K, Cadogan JIG, Rees CW (1992) Synthesis of 1,2-azaphosphetidines. J Chem Soc Chem Commun 3: 285-287.
- Yang KW, Feng LJ, Yang SK, Aitha M, LaCuran AE, et al. (2013) New β-phospholactam as a carbapenem transition state analog: Synthesis of a broad-spectrum inhibitor of metallo-β-lactamases. Bioorg Med Chem Lett 23: 5855-5859.
- Xu J (2020) Synthesis of 1,2-azaphosphetidine 2-oxides/sulfides. Chem Heterocycl Compd 56: 308-310.
- Fu X, Li X, Xu J (2021) Synthesis of β-Phosphinolactams from Phosphenes and Imines. Org Lett 23: 8733-8737.
- Laviós A, Sanz Marco A, Vila C, Muñoz MC, Pedro JR, et al. (2022) Metal-Free Diastereo- and Enan-tioselective Dearomative Formal [3 + 2] Cycloaddition of 2‑Nitrobenzofurans and Isocyanoacetate Esters Org Lett 24: 2149-2154.
- He XL, Wen YW, Li H, Qian S, He M, et al. (2023) Diastereoselective Synthesis of Dihy-drobenzofuran-Fused Spiroindolizidines via Double-Dearomative [3 + 2] Cycloadditions. J Org Chem 88: 493-503.
- Hou Y, Cui M, Zhang K, Chen L, Tian R (2022) Annulation of phosphole sulfides via [3 + 2] cycloaddition with nitrones. Org. Chem Front 9: 6606-6610.
- Liu Y, Ji Y, Li J, Liu X, Wang C, Zhang H, Tian R (2024) An Unexpected Access to Phospholene Fused β‑Phosphinolactams by the Reaction of α‑C2‑Bridged Biphospholes and Nitrones. Org Lett 26(41): 8747-8751.
- Nyulászi L, Hollóczki O, Lescop C, Hissler M, Réau R (2006) An aromatic-antiaromatic switch in P-heteroles. A small change in delocalisation makes a big reactivity difference. Org Biomol Chem 4: 996-998.
- Frisch MJ, Trucks GW, Schlegel HB (2010) Gaussian 09 (Revision B.01), Gaussian, Inc., Wallingford, CT.
- Stephens PJ, Devlin FJ Chabalowski CF, Frisch MJ (1994) Ab initio Calculation of Vibrational Absorp-tion and Circular Dichroism Spectra Using Density Functional Force Fields. J Phys Chem 98: 11623-11627.
- Becke AD (1996) Density-functional thermochemistry. IV. A new dynamical correlation functional and impli-cations for exact-exchange mixing. J Chem Phys 104: 1040-1046.
- Lee CT, Yang WT, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37: 785-789.
- Li X, Kong X, Yang S, Meng M, Zhan X, et al. (2019) Bifunctional Thiourea-Catalyzed Asym-metric Inverse-Electron-Demand Diels-Alder Reaction of Allyl Ketones and Vinyl 1,2-Diketones via Dienolate Intermediate Org Lett 21: 1979-1983.
- Krenske EH, Houk KN, Harmata M (2015) Computational Analysis of the Stereochemical Outcome in the Imidazolidinone-Catalyzed Enantioselective (4 + 3)-Cycloaddition Reaction, J Org Chem 80: 744-750.
- Lv H, Han F, Wang N, Lu N, Song Z, et al. (2022) Ionic Liquid Catalyzed C-C Bond Formation for the Synthesis of Polysubstituted Olefins. Eur J Org Chem e202201222.
- Zhuang H, Lu N, Ji N, Han F, Miao C (2021) Bu4NHSO4‐Catalyzed Direct N‐Allylation of Pyrazole and its Derivatives with Allylic Alcohols in Water: A Metal‐free, Recyclable and Sustainable System. Advanced Synthesis & Catalysi 363: 5461-5472.
- Lu N, Liang H, Qian P, Lan X, Miao C (2020) Theoretical investigation on the mechanism and enantiose-lectivity of organocatalytic asymmetric Povarov reactions of anilines and aldehydes. Int J Quantum Chem 120: e26574.
- Tapia O (1992) Solvent effect theories: Quantum and classical formalisms and their applications in chemistry and biochemistry. J Math Chem 10: 139-181.
- Tomasi J, Persico M (1994) Molecular Interactions in Solution: An Overview of Methods Based on Continu-ous Distributions of the Solvent Chem Rev 94: 2027-2094.
- Simkin BY, Sheikhet I (1995) Quantum Chemical and Statistical Theory of Solutions-A Computational Ap-proach, Ellis Horwood, London, UK.
- Tomasi J, Mennucci B, Cammi R (2005) Quantum Mechanical Continuum Solvation Models. Chem Rev 105: 2999-3093.
- Marenich AV, Cramer CJ, Truhlar DG (2009) Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J Phys Chem 113: 6378–6396.
- Reed AE, Weinstock RB, Weinhold F (1985) Natural population analysis. J Chem Phys 83: 735-746.
- Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital do-nor-acceptor view point. Chem Rev 88: 899-926.
- Foresman JB, Frisch A (1996) Exploring Chemistry with Electronic Structure Methods, 2nd ed., Gaussian, Inc., Pittsburgh, USA.
- Lu T, Chen F (2012) Multiwfn A multifunctional wavefunction analyzer. J Comput Chem 33: 580-592.
-
Nan Lu* and Chengxia Miao.Theoretical Investigation on [2 + 2]/ [3 + 2] Cycloaddition from Biphosphole and Nitrone Yielding Phospholene Fused β‑Phosphinolactam or Isoxazolidine. Acad J Gastroenterol & Hepatol. 4(1): 2024. AJGH.MS.ID.000580.
-
[2 + 2] cycloaddition; 1-phosphafulvene; biphosphole; nitrone; β-phosphinolactam; Iris Publishers; Iris Publishers Indexing Sites
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Track Your Article
Refer a Friend
Advertise With Us
Feedback
 a Creative Commons Attribution 4.0 International License. Based on a work at www.irispublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.irispublishers.com.
Best viewed in





